These substances mainly have a constant or uniform composition throughout. Density is the ratio between mass and volume as mass increases the volume also increases in the same ratio so density for every pure substance at any given temperature is always constant.
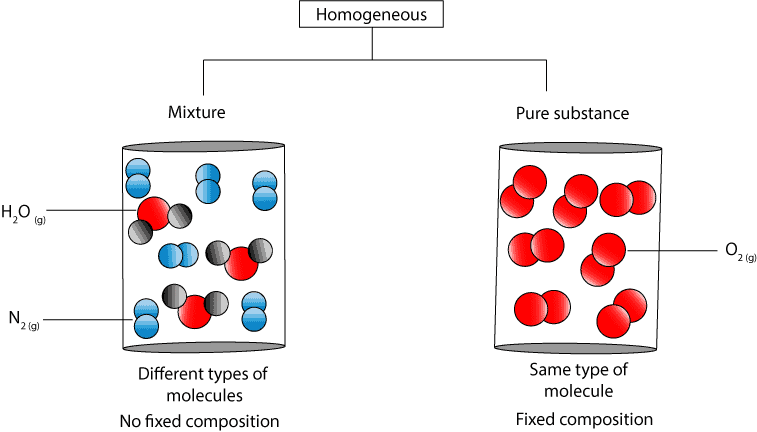
If A Substance Is Homogeneous Is It A Pure Substance
Every sample of a given substance has the same properties because a substance has a fixed uniform composition.

. Ratio of the density of the substance to the density of the reference substance which is usually water at the same temp. The density an object depends not only on the mass but also its volume ie. Examples of Pure Substances.
A cup of water cannot have the same mass as a drop of water. Density is the result of the relationship between mass and volume. A mixture of two or more phases of a pure substance is still a pure substance as long as the chemical composition of all phases is the same.
So even if you decrease the volume of a material assuming that it is homogenous throughout the density will remain the same. Density is very slightly less than specific gravity. I think that water has a relatively large change in density because of the formation of hydrogen bonds and that most materials undergo a much smaller change in density in going across their solid-liquid transition.
Even though there is more molasses mass in test tube A the molasses also takes up more space volume. For solids its also affected by the way atoms and molecules stack together. 1 Why does a pure substance always have the same density.
If the pure substance consists of different types of atoms then it is a compound. The substances have fixed boiling and melting points. 2 Can density be used to identify unknown substances in lab.
A pure substance can consists of atoms molecules or formula units. 3 You are given a square piece of aluminum foil and asked to calculate its density. A pure substance does not have to be of a single element or compound.
Density is simply the mass divided by volume. The density decreases from 09970 gmL to 09718 as it is heated. Specific gravity is the ratio of the density of a substance to the density of a standard usually for a liquid or solid and air for gas.
I assume that different conditions must include different temperatures and pressures so the answer is no Lets take pure. Describe the composition of a compound. Why does a pure substance always have the same density.
The density of a pure substance A whose atoms are in cubic closed packed arrangement is 1 gcc. It is really a matter of the structure of the solid and some solids have a very elaborate crystal structure while others have a less predictable structure. Does a pure substance always have the same density when measured under different conditions.
When can they not be used. Explain why the composition of an element is fixed. This means that it is the amount of the substance in.
Therefore if two objects had the same mass and volume then they would have the same density. If all the tetrahedral voids are occupied by B atoms what is the density in g cm 3 of resulting solidAtomic mass of A 3 0 gmol and atomic mass of B 5 0 gmol. Density is simply the mass divided by volume.
Another characteristic of properties is that the value of a property at the present is not dependent on the history of the substance. The mass and volume of a substance is directly proportional to the amount of matter making up the substance. By contrast if two objects had the same mass but different volumes then they would not have the same.
Volume and mass are extensive properties. An element has a fixed composition because it contains only one type of atom. This means that a pure substance will have a constant appearance colour density melting point and boiling point throughout the sample.
A pure substance usually participates in a chemical reaction to form predictable products. Also the density of a substance remains the same no matter what size it. Specific gravity is a ratio of two densities so it has no units.
Answer 1 of 2. Lets look at the density of water at 25 deg C and compare that to a higher temperature 80 deg C. The chart at right give the density in kgm 3.
User93237 Sep 28 2015 at 047 A first order phase transition must have a volume change. Why do pure substances always have a specific density. If two objects have the same mass do they have the same density.
Pure substances are mostly homogeneous in nature containing only one type of atoms or molecules. However because the density of pure water is so close to 1 09976 grams per cubic centimeter specific gravity and density are nearly the same value so long as the density is given in gcc. It also means that if you take a small sample of the original pure substance the sample will undergo the.
If the density of the liquid is greater than the density of the object then the object will float. This is because the mass and volume increase at the same rateproportion. Why or why not.
The density remains the same because cutting the object in half will divide the mass volume by the same amount. Often solids have a higher density than liquids because the atoms or molecules are packed more closely in an ordered fashion in the solid. Divide by 10 3 to get the density in gmL.
Therefore the spacing between those tiny particles that make up the molasses is constant does not change. Phases of a Pure Substance A pure substance may exist in different phases. For example carbon can take the form of graphite or diamond.
Because a pure substance indicates that it is exactly that a substance made of a specific combination of elements it will always have the same density because those elements can only take one form in order for it to be pure. This means that it is the amount of the substance in a specific unit of space. More order equals lower energy as it has less entropy.
However the density does not change. A pure substance can take many forms which dont have the same properties. From the concept map you can see that a pure substance can consists of different or the same type of atoms.
If the pure substance consists of the same type of atoms then it is an element. Second order phase transitions need not. Pure Water The density of liquid water is approximately 10 gmL.
The Factors That Affect Density Density depends on temperature and pressure.
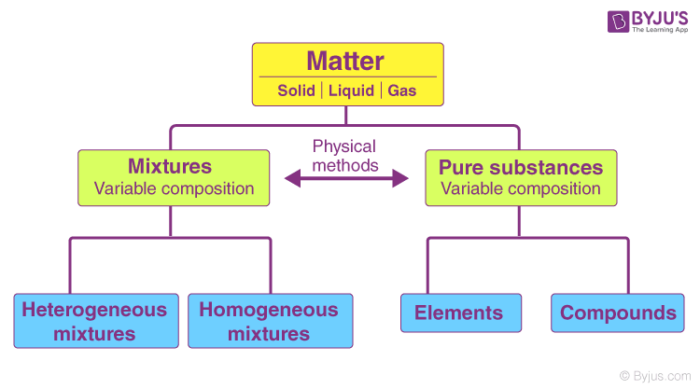
What Is Pure Substance Definition Examples Difference Between Pure Substance Mixture

Matter Can Be Classified As Mixtures Or Pure Substance Chapter 7 Ppt Download

0 Comments